The PDF data logger revolution
All-in-one PDF data loggers from ELPRO are providing new ways of monitoring temperature within the supply chain.
For a long time, temperature monitoring during storage and transport was a complicated matter. The challenges included lost data cables, different readout devices for each unit type, software that had to be updated regularly and new employees requiring training on how to prepare an evaluation report. With the invention of the PDF data logger, the world of temperature monitoring has been revolutionised by eliminating the requirement for tools, software and expensive software training at the destination.
"When ELPRO invented the PDF data logger in 2007, hardly anyone realised that this device would revolutionise an entire industry."
A brief history of temperature monitoring
Temperature control is a well-known problem and has its origins in the 19th century, when people began using ice to preserve goods while they were stored or transported. The use of ice meant there was a simple alert mechanism (if ice is present, then it's still cold enough), but since the invention of refrigerators, monitoring, logging and alerting have been important issues. In the 1980s, the pharmaceutical industry was caught off guard regarding cold chain management when it was discovered that enzymes and proteins could be used as drugs. Today, virtually every drug is declared temperature-sensitive. On the one hand, this has to do with the nature of modern drugs and, on the other, it is due to consumers' increasing awareness of the influence of temperature on the appearance and effectiveness of drugs.
To date, cold chain monitoring has been a tedious affair: each data logger usually needs their own reader, special data cables and, of course, dedicated software to configure readout and analyse data. Lost cables, readout devices with weak batteries and complicated software that requires regular updates are just a few of the challenges cold chain managers face. The standard solution was for the devices to be sent back to the sender for analysis. Only after this was completed could the drugs be released at the destination. It is easy to see that this procedure resulted in loss of time and money.
How does the PDF data logger work?
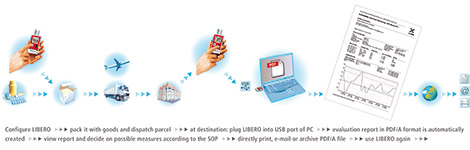
When ELPRO invented the PDF data logger in 2007, hardly anyone realised that this device would revolutionise an entire industry. The PDF data logger is actually based on a simple idea: all in one ' the data logger automatically generates an evaluation report no matter where it is and without requiring additional software, data cables or a readout device. This was made possible through the combination of a traditional data logger with a USB interface and modern microprocessors, which are able to compare the measured values with predefined criteria and prepare a PDF report that can be read out via the USB port of any computer without requiring additional software (USB memory stick principle). The steps in using a PDF data logger are always the same:
1. configure: define measurement interval and alerting criteria, or purchase a pre-configured device
2. start: activate the data logger and position it with the product
3. stop: remove the data logger from the product at the destination and stop the measuring period
4. readout: plug the device into a USB port to generate a PDF report.
 |
Application: cold chain
The most significant application of PDF data loggers is cold chain monitoring of commercial products and clinical trial shipments. Worldwide, the number of temperature-controlled shipments is estimated at several million annually; these estimates usually consider only the first stage of the entire cold chain, which extends from manufacturer, via local distributor, to pharmacy/hospital and finally the patient. After great efforts are made to bring a drug to the pharmacy in perfect condition, 'the last mile' has now become an issue: how do you ensure that the patient does not destroy the active pharmaceutical ingredient on the way home or through improper storage at home?
Requirements and challenges
Although the basic requirements and challenges are always the same (configure, start, stop, evaluate), there are important differences in each specific application. When shipping commercial products (those intended for sale), large volumes are generally transported on a regular basis to a known, small network of local distributors. When shipping products for clinical studies, however, small volumes are generally dispatched at short notice to an unknown and constantly changing number of destinations around the world. It is clear that different shipment modes pose different monitoring requirements. While a stable and inexpensive solution is preferred for commercial shipping, clinical trials demand a solution that is flexible and simple. Today it is inconceivable that a patient in a clinical trial in Africa should have to wait until the data logger has been sent back to Europe for evaluation.
"The PDF data logger is actually based on a simple idea: all in one - the data logger automatically generates an evaluation report no matter where it is and without requiring additional software, data cables or a readout device."
 |
LIBERO THi1 for temperature and relative humidity with its PDF report. |
Advantages and benefits of using a PDF data logger in the cold chain
The main advantages are the time savings and improved safety regarding product release at the destination. The enormous simplification of the process also brings cost reduction into play. The simplicity of the configuration procedure at the sender via
USB interface and the use of multiple alert zones present further benefits that have contributed to the device's success. In a few years, PDF data logger has become the industry standard and it is impossible to imagine cold chain management without it.
Outlook
Innovation and development continues. Trends and buzzwords often heard in this context are ambient monitoring, relative humidity, cold chain databases and automation.
Ambient monitoring
It is now well known that even drugs labelled 'store at ambient temperature' are sensitive to extreme temperatures. Furthermore, the number of drugs being developed in this segment is increasing. Few are aware when defining the requirements that it is generally more difficult and expensive to consistently maintain 15.25°C than 2.8°C. A PDF data logger can also contribute to a solution in this area, whether by the (measurement and) definition of ambient temperatures or by monitoring ambient temperature transports.
Relative humidity
There is increased concern regarding relative humidity during the transportation of pharmaceutical products. The most significant issues are the outsourcing of production to Asia (subtropical climate) and the desire to transport bulk products (such as open, unsealed drugs and agents). The principle is exactly the same, with the additional measurement of relative humidity and humidity values defined as alert criteria.
Databases
GMP requirements call for the storage of logger data for a number of years (until the expiry date). The management of this data is accompanied by the concern of costs. This issue has grown in importance over recent years, and is often concealed by the qualitative requirements of CAPA (continuous improvement programme). Since the raw data is embedded in a hidden area in the PDF report, it can easily be sent via email, then electronically read and evaluated. Managing logger data with an online cold chain database such as liberoMANAGER, is a simple and costeffective cloud-based solution for data archiving.
Automation
In order to further optimise cold chain monitoring, processes have to be automated to the greatest extent possible. Manual entries and inputs, which often lead to errors and mistakes, should be avoided. PDF loggers like the LIBERO PDF Logger and a number of software tools enable automation at the sender's and the recipient's ends. In this application, a PDF data logger is used as an information carrier. By including a dynamic library (DLL) in the client's logistics software or by using LIBERO SmartStart from ELPRO, users can feed additional shipment specific information into the device.

