|
"The objective of an adaptive design trial is to generate high-value clinical data to drive 'smarter' product development decision-making."
|
Innovation through design and execution of adaptive trials
Adaptive trials represent the major route through which innovative trial design is being integrated into product development strategies.
Adaptive design trials offer the greatest potential for changing the product development paradigm.
Creating efficiency and value - generation of high-value clinical data
The objective of an adaptive design trial is to generate high-value clinical data to drive 'smarter' product development decision-making. This is done through the implementation of an interim analysis step (or steps) at which one of several pre-planned trial design adaptations are executed. It is important to note that trial validity must be preserved through the use of appropriate statistical methodologies developed for adaptive design studies - and embodied in agency guidance. Design adaptations enable a much more efficient trial to be conducted and, if implemented throughout a development programme, can save time, money and increase the probability of success at phase III.
The ethical imperative
Sponsor companies are duty bound to protect patients from treatments and interventions that are ineffective, unsafe or cause harm. Pre-planned mid-study adaptations afforded by appropriately designed adaptive trials provide the best mechanism to optimise treatment and safeguard the welfare of patients, without compromising trial integrity and validity. In the majority of cases conventional trials do not permit this and as such should only be used where an adaptive approach is impractical.
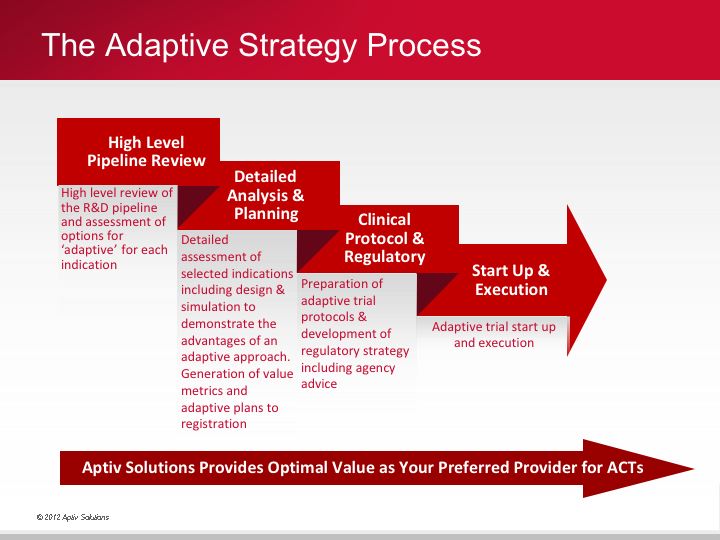
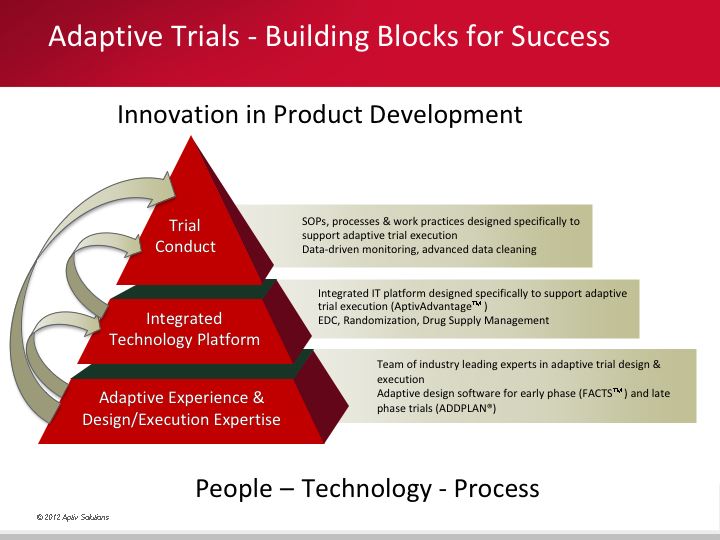
Critical success factors for innovative adaptive trials
Successful execution of adaptive trials requires three critical components - people, technology and process:
- people: expertise and experience in design and implementation of adaptive trials
- technology: an integrated execution platform
- process: SOPs and work practices that support advanced data cleaning and data driven monitoring.
|
 |
|
Adaptive trials represent the major route through which innovative trial design is being integrated into product development strategies. They offer the greatest potential for changing the product development paradigm. |
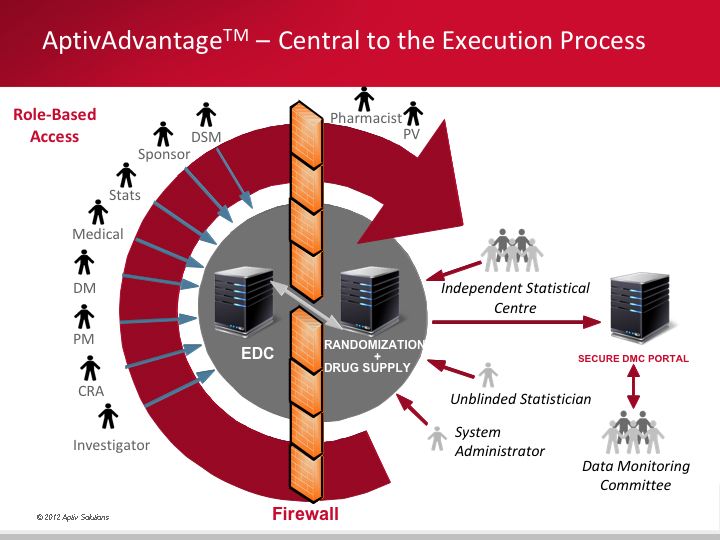
AptivAdvantage™ is a new integrated technology platform specifically designed for the execution of adaptive trials, although it is finding significant utility in the efficient delivery of traditional trials. |
The Aptiv Solutions Innovation Center
Aptiv Solutions created the Innovation Center to improve the conduct of drug development using adaptive trials. The Innovation Center comprises the world's leading experts in novel design methodology and adaptive trial execution, who are driving innovation in product development and are available to assist sponsor companies in trial design and execution.
|
"Aptiv Solutions' Innovation Center comprises the world's leading experts in innovative design methodology and adaptive trial execution, who are driving innovation in product development."
|
|
Complex adaptive trials are now feasible
Through the use of AptivAdvantage™, sponsor companies have the capability to implement complex adaptive design trials employing novel design methodologies - enabling them to learn significantly more about their development product and make more-informed decisions on subsequent development.
Population enrichment methodologies for confirmatory studies have been recently embodied in fully validated adaptive design software called ADDPLAN® PE. The application of this innovative adaptive approach will dramatically enhance the ability of sponsors to realise the true potential of targeted therapy with the ultimate objective of improving patient care and reducing healthcare spending.
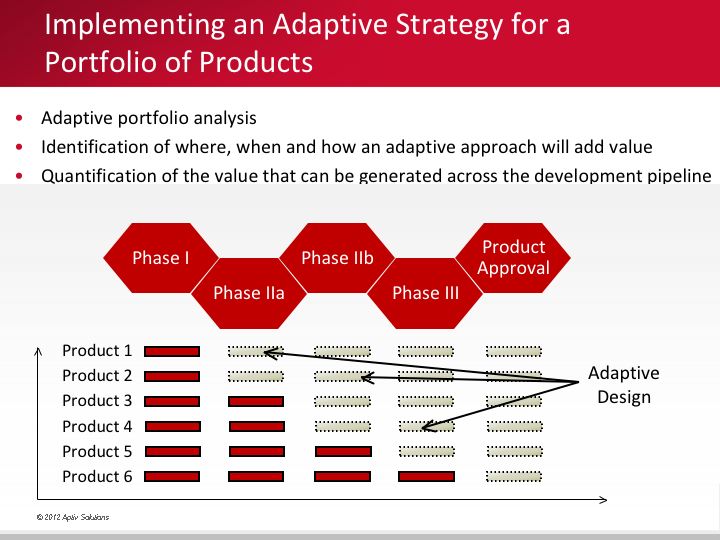
Implementing an adaptive strategy for your company's product portfolio
Devising an innovative trial strategy across a portfolio of products, whether for a single therapeutic area or across several disease states, is a critical step in understanding the value that adaptive design can create.