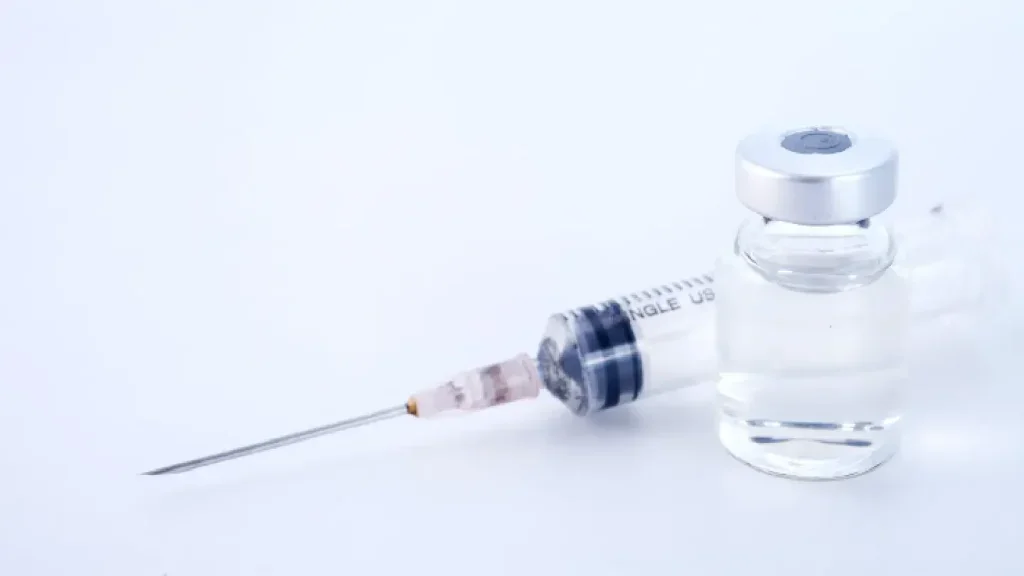
Sobi secures FDA approval for Gamifant to treat MAS in Still’s disease
The FDA approval was based on pooled data from two studies, including a Phase 3 trial, in which 54% of patients achieved a complete response.
A leading resource for the Pharmaceutical industry since 2002
The FDA approval was based on pooled data from two studies, including a Phase 3 trial, in which 54% of patients achieved a complete response.

Chemspec Europe 2025 returns to Cologne with expanded exhibition space, enhanced digital tools, and a diverse line-up of industry leaders. This premier event will showcase cutting-edge solutions across key sectors,...
Seal the deal
Excipient evolution shaping pharma innovation
Solving the EDDO guidance puzzle
AI-driven delivery