All articles by Pradeep Bairaboina
Deep Apple, Novo Nordisk sign $812m deal for cardiometabolic drug development
The partnership will leverage Deep Apple’s proprietary drug discovery platform, which integrates machine-learning-driven virtual screening with structural biology facilitated by cryo-electron microscopy.
SpliceBio secures $135m to advance gene therapy for Stargardt disease
The funds will support the clinical development of SpliceBio’s leading gene therapy candidate, SB-007, for Stargardt disease.
Simtra BioPharma Solutions and MilliporeSigma announce strategic alliance
The partnership brings together the strengths of two leaders and creates a turnkey offering for biopharmaceutical companies seeking ADC and bioconjugation, linker/payload manufacturing, drug product formulation development and fill-finish capabilities.
China approves Zymeworks’ Zanidatamab for HER2-positive biliary tract cancer
The approval is for the treatment of patients with previously treated, unresectable, or metastatic HER2-positive biliary tract cancer
Blueprint Medicines and VantAI to advance novel therapies with expanded agreement
The collaboration will focus on induced proximity drug discovery, tackling targets previously deemed “undruggable” by traditional therapeutics
BioNTech secures UK government grant to boost R&D in innovative medicines
The initiative aligns with the UK government’s strategy to bolster its life sciences sector and foster innovation in medical science
Quoin Pharmaceuticals announces EMA grants Orphan Drug Designation for QRX003 for the treatment of Netherton Syndrome
The company continues to advance QRX003 in late-stage clinical trials in Netherton Syndrome patients
Regeneron to acquire 23andMe’s assets in $256m deal
Regeneron plans to acquire 23andMe’s Personal Genome Service, Total Health, and Research Services divisions, as well as its Biobank and related assets
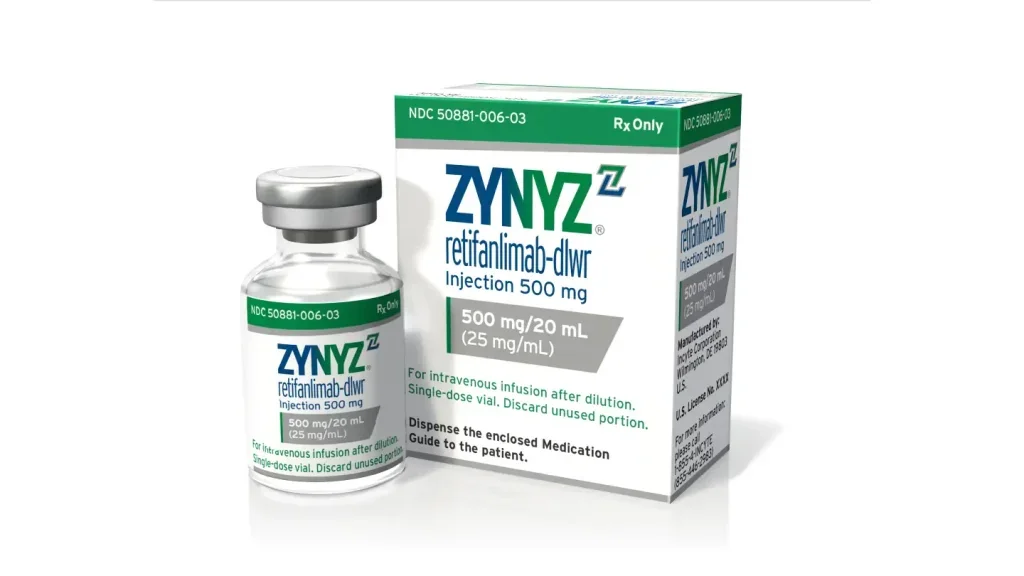
US FDA approves Incyte’s Zynyz for advanced anal canal cancer treatment
The approval of Zynyz as a monotherapy was based on the POD1UM-202 study, which reported an objective response rate of 14% and a disease control rate of 49%
Sarepta’s Elevidys receives conditional approval in Japan for DMD
The approval is based on the efficacy and safety data from clinical studies, including long-term functional results from the global Phase 3 EMBARK study