White Papers
CMC Process development and risk management in peptide manufacturing
Developing peptide and oligonucleotide active pharmaceutical ingredients (APIs) demands a precise balance of efficiency and attention to compliance. To meet …
Connecting cold chain solutions to the world
Air Canada Cargo's commitment to delivering cold chain logistics across the globe.
What does it take to stay on top in pharma air freight?
In taking on his new role, Joel Wobma, global head of pharmaceutical logistics at Air France KLM Martinair Cargo (AFKLMP), …
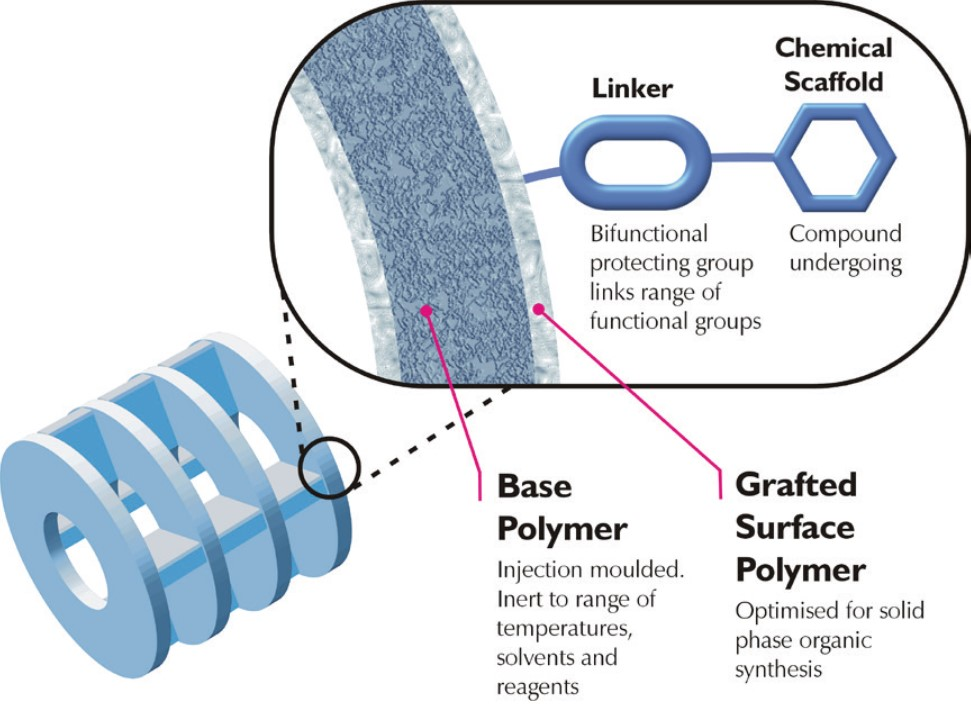
Simplifying small molecule synthesis
Keeping up with the modern-day demands of pharmaceutical research has necessitated advancements in rapid production of small molecules and peptides. …
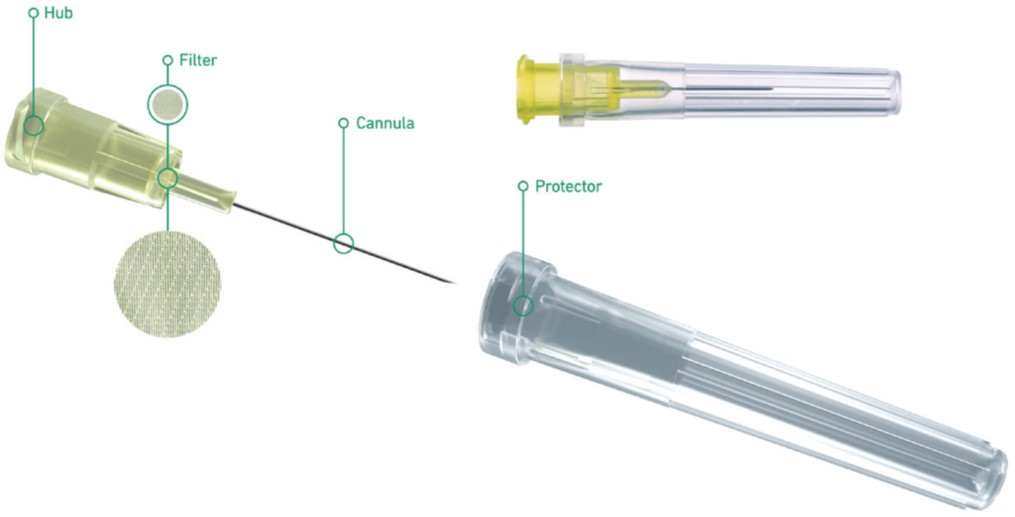
Bringing clarity to ophthalmics
Terumo launches its Terumo Injection Filter Needle, a first step of the INFINO™ – Development Programme, to extend the choice …
Leveraging the ‘D’ in CDMO to enhance innovation
Contract development and manufacturing organisations (CDMOs) can play a vital role in the R&D of medical devices, as well as …
Shaping the future of home drug delivery
Greg Moakes, EVP, new business development for the Device Technology unit at LTS, discusses the rapid growth of the on-body …
Making review by exception the rule
The pharmaceutical industry has long struggled to boost the efficiency of its batch review process. One solution that companies have …
Drying processes for oncological products
LAST Technology is developing an innovative ATEX drying technology for processing highly potent API containing solvents.
Expanding for the future
Modern packaging manufacturing isn’t easy – especially for companies with worldwide ambitions. Medical Device Development talks to insiders at Oliver …
Bridging silos in clinical trials data
The success of clinical trials relies on integration of various functions, yet silos between Data Management, Biostatistics, Data Standards, and …
A game-changer for clinical trial design
Cytel, a leading provider of cutting-edge analytical solutions, quantitative methods, and innovative statistical software for the life sciences industry, has …
What happens when data is better prioritised?
Drug development is rife with complexities. Success hinges upon managing them effectively. Data is the ultimate deliverable of any study, …
Plotting a course toward content automation
With Generative AI reshaping content automation, organisations must ensure they select technology that is scalable and fit for purpose. Here …
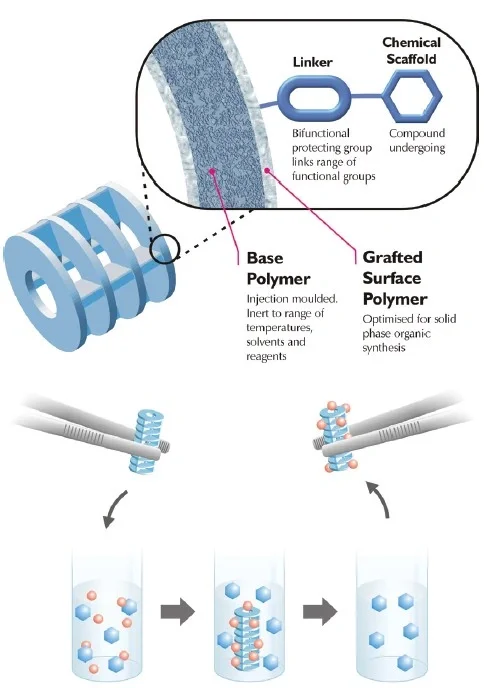
Simplifying small molecule synthesis
Keeping up with the modern-day demands of pharmaceutical research has necessitated advancements in rapid production of small molecules and peptides. …