All articles by Swagath Bandhakavi
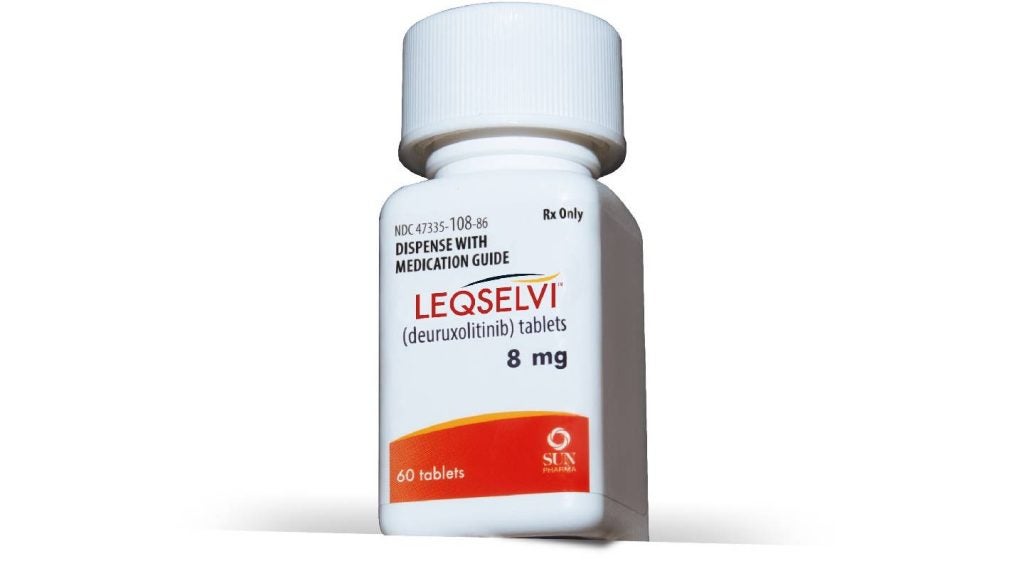
Sun Pharma launches Leqselvi in the US for severe alopecia areata
Sun Pharma’s new oral selective inhibitor, targeting Janus Kinases JAK1 and JAK2, is now available for prescription throughout the US.
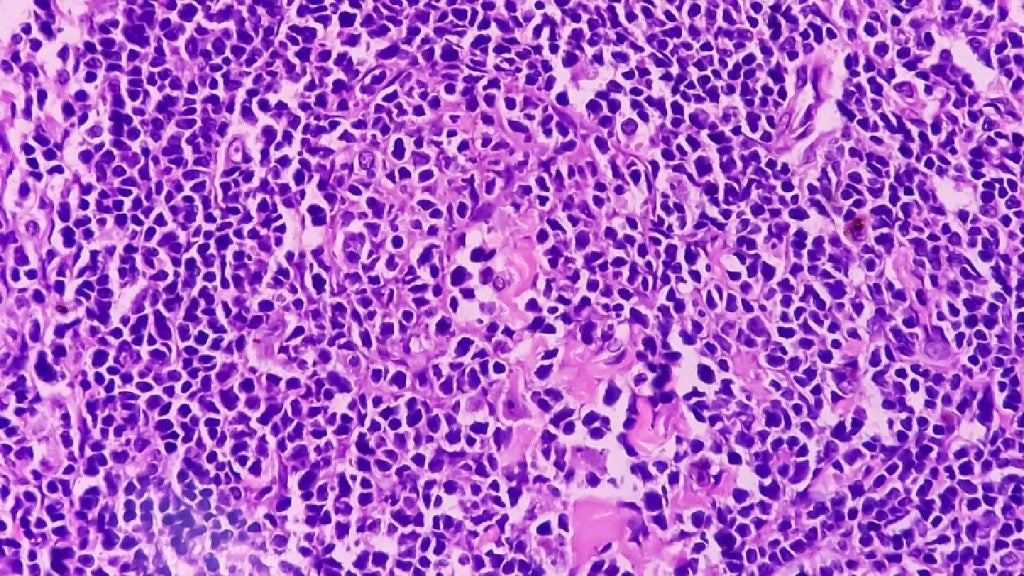
Imugene’s azer-cel shows promise in treating relapsed DLBCL
The company reported that six of seven patients reached complete remission, resulting in a 55% complete response rate.
FDA approves Lilly’s revised Alzheimer’s dosing regimen for Kisunla
The update introduces a new titration dosing method to reduce side effects without affecting amyloid plaque reduction efficacy.
Swissmedic approves Norgine’s IFINWIL for paediatric HRNB
IFINWIL inhibits ornithine decarboxylase, an enzyme crucial for producing polyamines that support tumour growth.
GSK closes $2bn acquisition of efimosfermin from Boston Pharmaceuticals
Efimosfermin is in phase III as a monthly subcutaneous treatment for MASH, an advanced steatotic liver disease.
European Commission fines Alchem for breaching antitrust rules
The EC found Alchem involved in cartel activities concerning SNBB, a crucial component for Buscopan production.
Alembic acquires Utility Therapeutics to bolster antibacterial portfolio
The acquisition grants Alembic access to PIVYA, the first FDA-approved antibiotic for uncomplicated urinary tract infections in two decades.
Dizal’s Zegfrovy wins FDA approval for targeted NSCLC treatment
Zegfrovy is an oral irreversible EGFR inhibitor targeting diverse EGFR mutations with selectivity for wild-type EGFR.
Merck completes $3.4bn acquisition of SpringWorks Therapeutics
SpringWorks provides Merck with a range of therapies for rare tumours, addressing areas with limited treatment options.
AbbVie to expand RNA capabilities with $2.1bn acquisition of Capstan Therapeutics
As part of the deal, AbbVie will acquire Capstan’s candidate CPTX2309, in Phase 1 trials, and its tLNP platform for targeted RNA delivery.