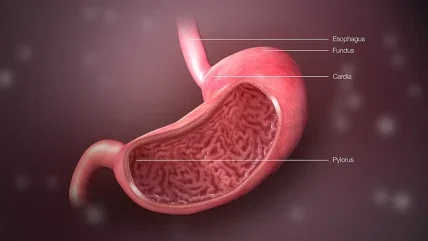
Vanda Pharmaceuticals has announced that the US Food and Drug Administration (FDA) declined its New Drug Application (NDA) of tradipitant for treating gastroparesis symptoms.
The company’s tradipitant application included details of two placebo-controlled studies which were previously published in peer review journals.
It also included exposure response data from a large open label study and experience of dozens of patients treated in an expanded access programme as evidence.
However, the FDA Complete Response Letter (CRL) suggested Vanda to conduct additional studies.
In a statement, Vanda said: “Despite this disappointing action by the FDA, Vanda believes that the tradipitant application has met the substantial evidence of efficacy standard with a favourable benefit risk profile for the treatment of patients with gastroparesis.
“While Vanda has repeatedly requested that the FDA convene an expert advisory committee to review the application and advise the Commissioner on the approvability of this application, the FDA has refused to do so.
“A number of patients currently treated with tradipitant have filed a Citizen Petition urging FDA to approve tradipitant for the treatment of gastroparesis.”
The company also claimed that FDA’s decision was delayed by more than 185 days and failed to meet the requirements specified by the Food Drug and Cosmetic Act (FDCA).
Gastroparesis condition shows down gastric emptying and causes severe nausea, vomiting, and difficulty finishing a normal meal among other symptoms.
Vanda noted that it will continue to pursue the marketing authorisation for tradipitant. Later this year, it also plans to file a separate NDA for tradipitant for the prevention of vomiting in motion sickness.
Last year, Vanda bought the US and Canadian rights to relapsing multiple sclerosis (RMS) therapy Ponvory (ponesimod) from Actelion Pharmaceuticals (Janssen).